Catheter Leak Testing
Uson offers equipment and solutions for effective catheter leak testing using our Sprint mD multi-function leak and flow tester.
The Challenge
Fast-flush drug delivery device leak testing is a complex process. Drug delivery devices typically have an inlet and outlet port, a flow restrictor, and a valve to bypass the restrictor. Makers and suppliers of these devices must ensure correct operation and integrity.
Challenge #1: Find all the right equipment to measure very low flow, high-flow, valve re-seating, valve leakage, and body leaks in the product.
Challenge #2: Organize the equipment into one integrated test. Sometimes accomplished with computers and programmable logic controllers, this can be a formidable task. The resulting group of equipment can be bulky, difficult to calibrate, hard to operate and a burden to keep maintained.
The Sprint iQ is the only off-the-shelf answer to fast-flush drug delivery device leak testing. No other tester in the world is manufactured to meet the demands of this specific product test. Incorporating a pressure sensor, two precision mass flow transducers, valving and logic control; the Sprint iQ handles the complex device testing routing in one small and easy-to program tester. With the press of Sprint iQ’s start button, the six-step test performs:
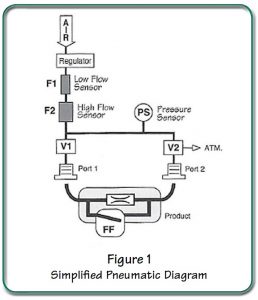
The Sprint iQ tester works like this:
Uson provides leak and flow testing solutions for the medical device manufacturing industry. In addition to extensive experience with fast-flush delivery device applications, Uson has assisted manufacturers with testing solutions for catheters, well plates, breathing tubes and many more. Our team. Contact us today and let's talk about your next project.
Uson offers equipment and solutions for effective catheter leak testing using our Sprint mD multi-function leak and flow tester.
Uson offers medical device leak testing solutions for testing breathing tubes and CPAP tubes using our Optima vT leak and flow tester.
Uson offers medical device leak testing solutions for testing well plates using our Sprint mD leak and flow tester.
Industries